
About Hydrogen Energy
Hydrogen is the most abundant element in the universe. Hydrogen is an energy carrier that can be used to store, move, and deliver energy produced from other sources (US DOE, 2022). Because of its unique properties and natural abundance, hydrogen has great potential to provide a clean, renewable source of energy to power things like heavy manufacturing machinery, industrial equipment, and medium and heavy-duty trucks.
Hydrogen Production
Since hydrogen doesn’t typically exist in nature by itself, hydrogen must be produced from the compounds that contain it.
Hydrogen fuel can be produced through several methods. One way to get hydrogen fuel is from water. Water is made up of hydrogen and oxygen, and when split through a simple reaction, it releases free hydrogen molecules. This process is known as electrolysis. Another common method of producing hydrogen fuel is by creating a reaction with steam and a carbon fuel (like natural gas or biogas). There are also solar-driven and biological processes that can produce hydrogen fuel.
The different types of hydrogen production are expressed as the Hydrogen Color Spectrum (NARUC, 2021), which is depicted here.
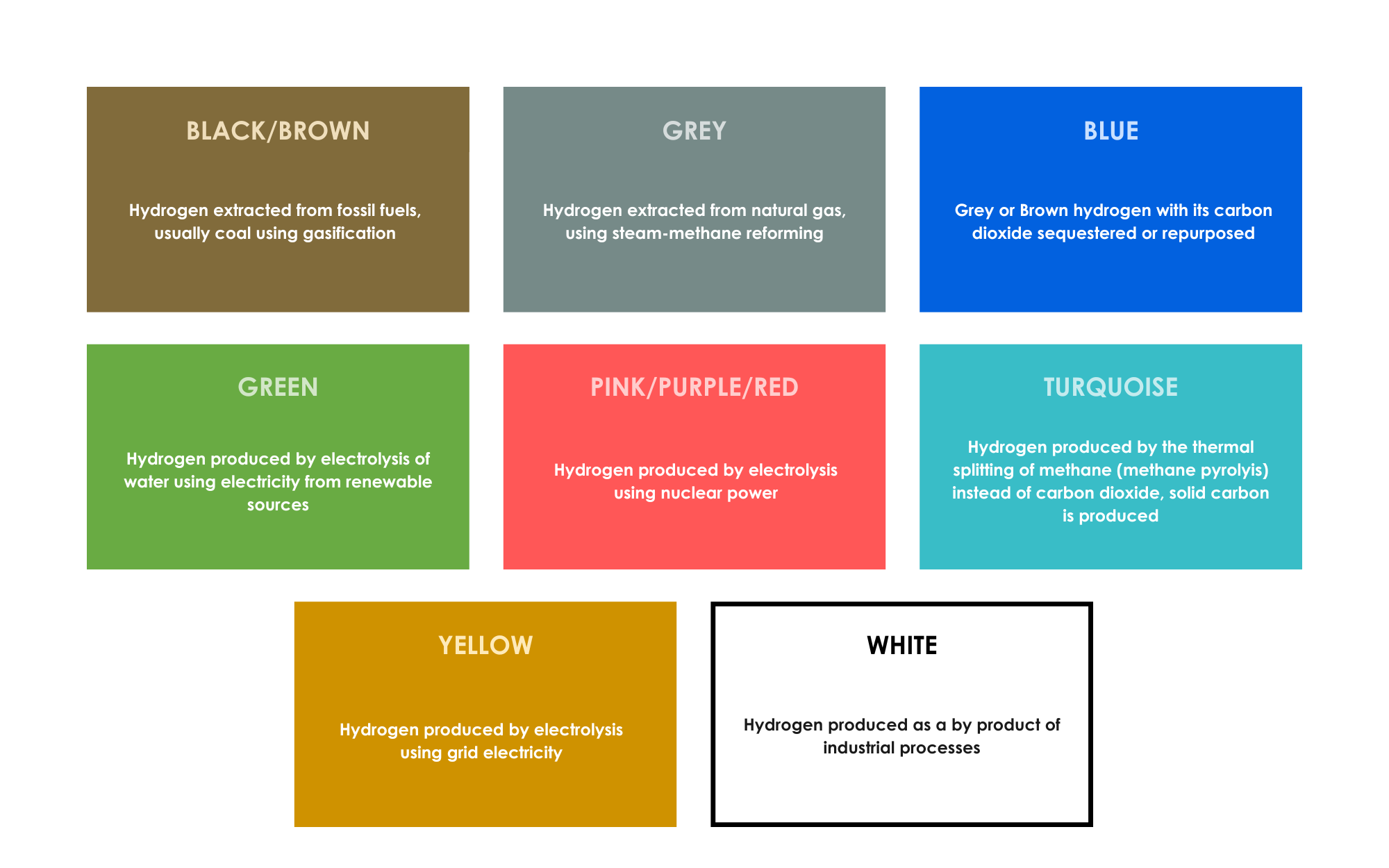
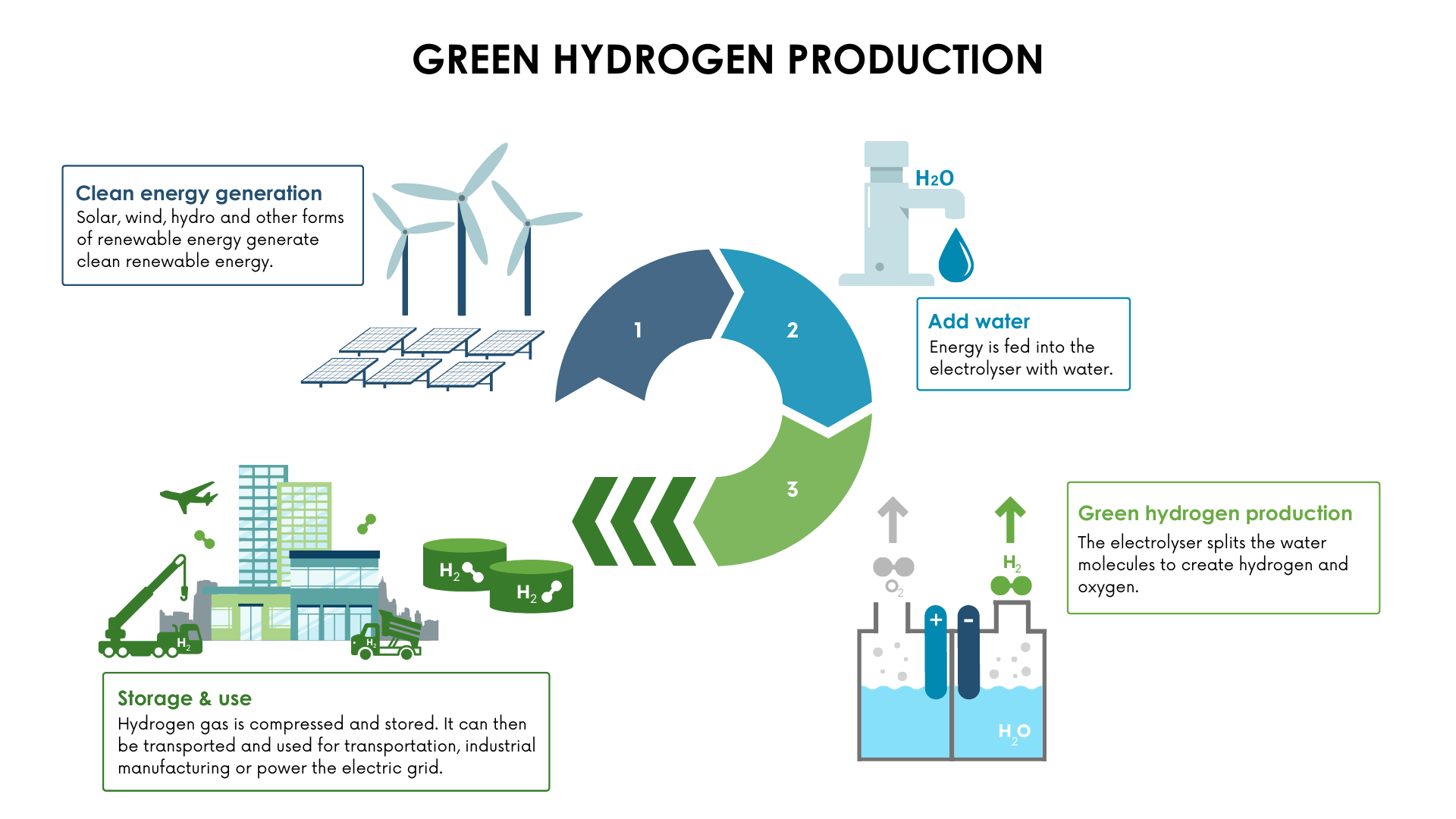
Hydrogen produced from the electrolysis of water using electricity generated from renewable sources is referred to as “Green hydrogen.” Utilizing green hydrogen offers the greatest potential reductions in greenhouse gas emissions because it releases zero emissions.
The Department of Energy (DOE) is currently developing a Clean Hydrogen Production Standard.
Hydrogen Fuel Cells
Hydrogen fuel cells are one way that hydrogen power can be harnessed. Fuel cells can be thought of as batteries that only operate when pure hydrogen is supplied to them and are used in a wide range of applications such as transportation, industrial machinery, and generate and store electricity for the grid. When hydrogen fuel is supplied to the fuel cell, an electrochemical reaction takes places between the hydrogen and oxygen (taken directly from the air) converting chemical energy into useable electrical energy.
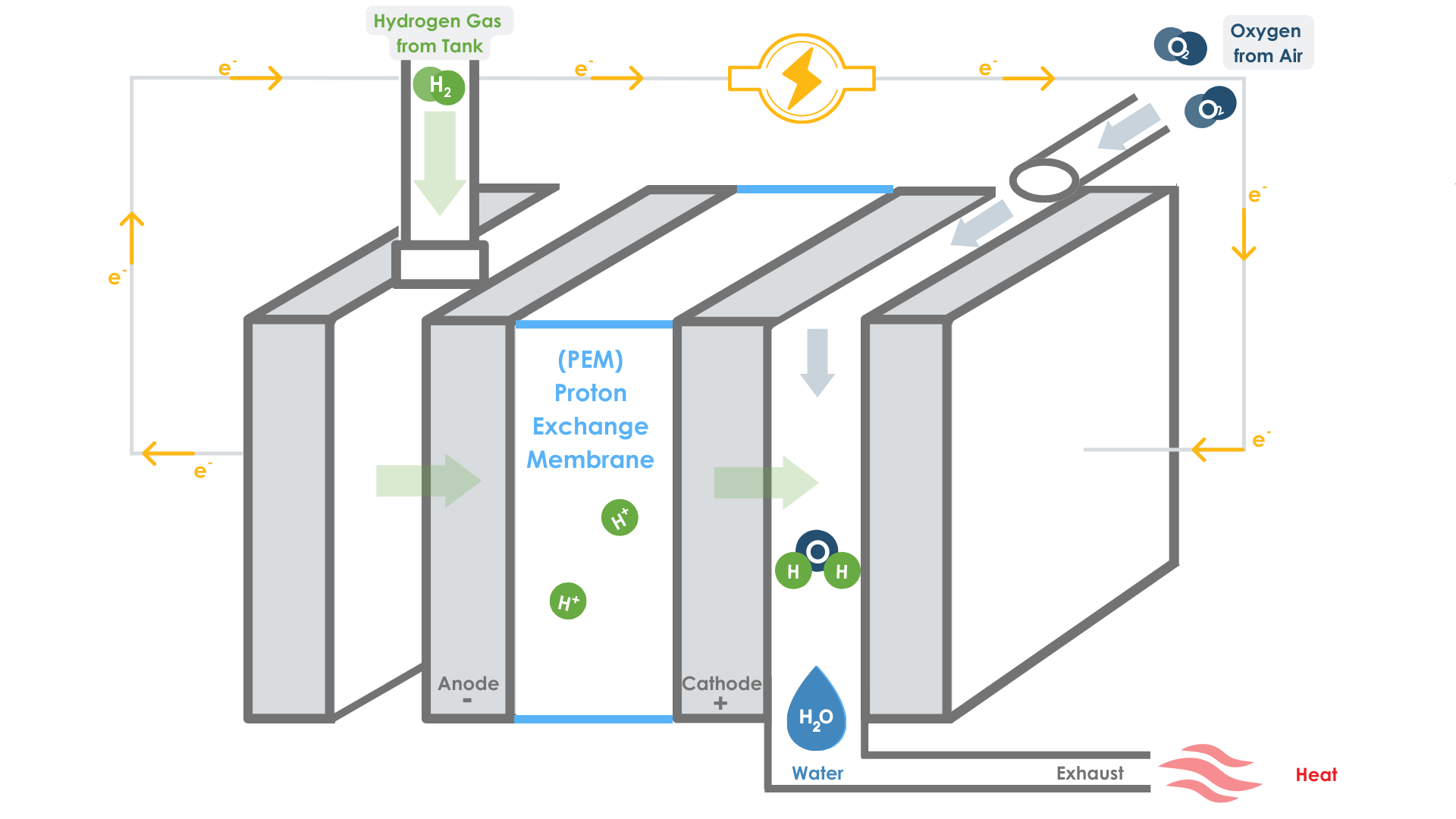
The proton exchange membrane (PEM) fuel cell, (shown in the diagram) is the primary type of fuel cell being designed for electric vehicles. It consists of two electrodes—a negative electrode (anode) and a positive electrode (cathode)—sandwiched around an electrolyte.
Hydrogen gas that is pumped in from a tank reacts with a platinum anode, which ionizes the gas into: positive ions (hydrogen protons) and negative ions (electrons). The protons (H+) can pass through the proton exchange membrane (PEM), while the electrons (e-) are forced to travel around the membrane through a circuit, generating the electricity that runs the car’s systems.
Once the protons pass through the membrane, and the electrons make it through the circuit, they combine in a reaction with the oxygen to produce the “waste” products: water and heat. Remaining water vapor may drip out the tailpipe as exhaust.
Individual fuel cells are then stacked together to create a higher voltage that can provide electric power for longer travelling distances (NOVA, 2005). Other arrangements and designs of fuel cells can be used in systems that power heavier-duty transportation, industrial machinery, and generate and store electricity for the grid.
Hydrogen Power's Potential
Fuel cell systems generate electricity through an electrochemical reaction, as opposed to combustion. The byproducts of hydrogen fuel cell systems are only electricity, water, and heat. The power systems that use these cells are clean, efficient and reliable, and do not need to be constantly recharged, as do battery systems. Unlike combustion generators, fuel cell systems emit no greenhouse gases or criteria air pollutants, allowing for improved local air quality. A fuel cell system will continue to produce energy if the initial fuel source (natural gas, biogas, hydrogen, etc.) is continually supplied. If the initial fuel source used to produce the hydrogen is renewable energy (i.e., wind turbines or solar arrays provide the electricity needed to produce hydrogen fuel from water), then the entire energy process releases zero carbon emissions.
End Uses of Hydrogen Energy
Clean hydrogen energy technologies have the potential to replace fossil fuels in sectors of New Jersey’s economy that are difficult to decarbonize using battery electric technologies. Critically, clean hydrogen energy has potential to be utilized for and eliminate emissions from:
- Medium and heavy-duty transportation
- Heavy duty airport equipment
- Heavy duty industrial manufacturing processes and equipment
- Back-up electric power generation
- Electric grid power production
- Electric grid energy storage
Decarbonizing these activities with clean hydrogen has the potential to improve local air quality in New Jersey’s overburdened communities, stimulate new job growth, and develop a workforce dedicated to a clean hydrogen economy that paves the way for a just transition.
References
Edwards, P.P., David, W. I. F., Kuznetsov, V. L.. 2007. Hydrogen energy. Philosophical Transactions of the Royal Society A. Pages 1043–1056. doi:10.1098/rsta.2006.1965.
National Association of Regulatory Utility Commissioners (NARUC). 2021. Coal and Carbon Management Guidebook: Coal-to-Hydrogen Opportunities and Challenges.
NOVA – PBS (2005). How Hydrogen Fuel Cells Work. PBS Online, WGBH, and the Corporation for Public Broadcasting.
Office of Energy Efficiency and Renewable Energy (2022). Hydrogen Production. Unites States Department of Energy.