In This Report:
Contact Us:
Environmental Trends Report
NJDEP, Division of Science and Research

Red Knot
Background
Overview
The red knot, Calidris canutus rufa, is an attractive migrant shorebird that stops by Delaware Bay each year to gorge on historically abundant and fat-rich horseshoe crab eggs. Arriving in their full breeding plumage, red knots can be identified by their straight black bill, a brilliant rusty red breast, and a patterned back of black, brown, and gray that provides camouflage when nesting. The species is listed as endangered in New Jersey and threatened at the federal level.1,2 Their remarkable life cycle involves one of the longest migrations in the animal kingdom. After journeying from as far away as the southern tip of South America, flocks of red knots arrive on the shores of Delaware Bay in early May to feast on horseshoe crab eggs before continuing to their Arctic breeding grounds. Some red knots travel more than 9,300 miles to reach their destination each spring. Figure 1 illustrates the migration path of the red knots that stop over in the Delaware Bay. After breeding, red knots and other shorebird migrants come back through New Jersey in mid-July through September on their annual journey back to wintering grounds in South America.3
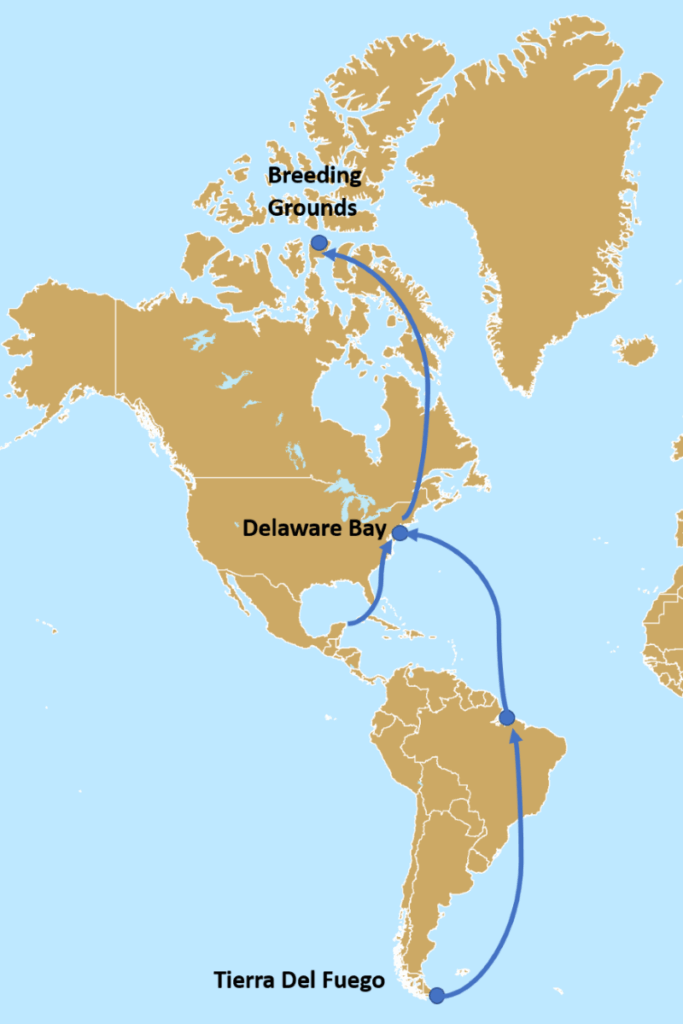
Figure 1. Red Knot Migration Path4
Relationship with Horseshoe Crabs
Red knot survival and productivity is closely tied to the productivity of spawning horseshoe crabs in Delaware Bay. Horseshoe crabs dig into the sand (5 to 30 cm deep) to lay clusters of eggs (3,000-5,000 eggs/cluster). Red knots and other shorebird migrants rely on eggs exposed by the digging action of abundant spawning crabs laying eggs in successive spawning events. To create new nests, crabs inadvertently dig up and expose previously laid eggs. If crab numbers drop to a low density, then eggs remain largely buried and unavailable to most shorebirds. Red knots have been observed feeding on mussel beds on the Atlantic Coast marsh in spring, but as a food resource, hard-shelled prey takes longer to metabolize into fat. Horseshoe crab eggs are easily digested and quickly metabolized into fat during the brief May stopover period (12-14 days). However, unregulated horseshoe crab bait harvesting in the 1990s sharply reduced crab populations, which has negatively impacted red knot populations. When crab eggs were more abundant, red knots could gain 8 to an astounding 15 grams of weight per day. Rapid weight gain is especially important for red knots migrating from greater distances (e.g., from Tierra del Fuego) that are more time constrained while on the Delaware Bay stopover. Horseshoe crab egg abundance has been reduced to less than one-tenth of the observed estimates from the 1980s, impacting numerous species. But long-distance migrants, especially red knots, have been the most drastically impacted.

Red knots and other shorebirds stop over in Delaware Bay to gorge on horseshoe crab eggs (Photo by NJDEP Fish & Wildlife)
Importance of Delaware Bay in Spring
Delaware Bay is a key migratory stopover for six Arctic-breeding shorebirds: the ruddy turnstone, the sanderling, the semipalmated sandpiper, the dunlin, the short-billed dowitcher, and the red knot. Of these migrants, the red knot is the largest and stockiest. The Bay has the largest spawning population of horseshoe crabs in the Western Hemisphere. Because horseshoe crab eggs are a fat-rich food, they have been a reliable resource for birds to rapidly increase body weight, which facilitates the completion of their journey to the Arctic and the prospects of successfully breeding. Packing on weight for long flights is a critical need for shorebird migrants during spring and fall migrations. Sufficient weight gain is statistically linked to higher adult survival and Arctic nesting productivity in red knots.
On Delaware Bay in the spring, red knots depend primarily on horseshoe crab eggs and, therefore, are frequently found in areas of dense horseshoe crab spawning, usually sandy beaches with gentle slopes and minimal wave action.5 Intertidal sand and mudflats along the Atlantic Coast are critical spring foraging areas in years when horseshoe crab spawning is curtailed by cold water or storms. When they arrive on the bay, red knots have little or none of the fat they accumulated while in South America. Many birds will burn off fat and muscle to reach the bay arriving at or below fat-free weight (130 grams). Baker et al. (2004) determined that knots departing Delaware Bay weighing at least 180 grams exhibited better annual survival rates.6 Red knots should reach a minimum weight of about 180 grams by the end of May in order to reach Arctic nesting grounds in adequate breeding condition.
Major concentrations of red knots are typically found along the southern Delaware Bay beaches of Cape May County, which includes Reed’s Beach south to Norbury’s Landing spanning the US Fish and Wildlife Service’s Cape May National Wildlife Refuge (NWR), as well as along northern Delaware Bay beaches of Cumberland County (Dennis Creek Wildlife Management Area (WMA), Egg Island WMA, and Fortescue). Red knots, and 30 other Arctic-breeding shorebirds, can also be found in summer and fall using Atlantic coastal marshes and exposed tidal sand and mudflats (Cape May to Barnegat Inlet) and high-energy beaches of the Cape May peninsula (e.g., Two-Mile Beach Unit of Cape May NWR, North Wildwood, Hereford Inlet, Stone Harbor Point, Avalon and Strathmere), Atlantic County (Malibu Beach WMA, North Brigantine Natural Area, Edwin B. Forsythe NWR), Ocean County (Holgate Unit of Forsythe NWR), and Monmouth County (Sandy Hook National Seashore).
Continuing Their Journey
By late May to early June, with fat resources accumulated in Delaware Bay, the birds leave for the Canadian Arctic tundra. They arrive in early June often facing frozen conditions and substantial snow cover. Red knots make their nest scrapes in snow-free sections of barren rocky areas, well inland from coastal wetlands. After laying four eggs, the male and female share incubation and rely on body fat, accumulated in the Delaware Bay, for 1-2 weeks until snow melt and insect emergence. Invertebrate prey may be unavailable until the third or fourth week of June, when the eggs begin to hatch. Females begin migrating south by mid-July, followed by males and then hatching-year offspring. Significant numbers of long-distance red knots stop over in New Jersey on southbound migration starting in mid-July and remain for three to six weeks to make large weight gains before departure for South America. Some portion of the hemispheric population remains in New Jersey for two months or more to molt flight feathers (short-distance migrants). Abundant intertidal prey including small clams, mussels, and marine invertebrates, along beaches, inlets and back bays, allow long-distance migrants to make large weight gain prior to trans-Atlantic flights to South American wintering areas. Short-distance migrants remain on New Jersey’s Atlantic Coast for more than two months to undergo flight feather molt before sub-freezing weather in late-November and December pushes birds to southeast US and Caribbean wintering areas.
Survey Description
In 1985, NJDEP Fish and Wildlife (NJFW) designed a long-term aerial survey to assess annual trends in bird relative abundance on bayshore beaches. The aerial survey is conducted by airplane in May and June, with the goal of surveying once per week for six weeks. A single day peak count from each survey year is a useful metric as a population index for assessing change over time. The peak count is not a true population estimate as it does not account for the turnover of birds during May, but it is an important index of abundance and annual foraging conditions. Notably, peak count data collected in the aerial surveys are only a snapshot in time. Depending on the conditions at that time, the results can vary widely. For example, NJFW has documented high variability on which side of the Delaware Bay is utilized by red knots. From 2009-present, in addition to the aerial surveys, concurrent ground surveys have been conducted to supplement or corroborate aerial data.
Researchers have captured knots on the Bay annually from 1997-present in order to band them and collect several biometric measurements, including weight. Each year red knots, ruddy turnstones, and sanderlings are banded with field-readable “flag” bands. Resighting these coded flag bands identify individuals, which provides data for population estimation. Weight data is used to track the rate of weight gain for shorebird flocks in the Bay. Further, the number of red knots that meet or exceed 180 grams in late May can represent the proportion of the peak count of birds that will likely have successful reproductive outcomes.

Red knots on beach with one banded leg (Photo credit: Mark Peck)
Status and Trends
Peak counts of Red Knots
The red knot stopover population in Delaware Bay has declined relatively rapidly since shorebird surveys first began.7 From 1986 to 2024, the peak count of red knots ranged from 6,880 to 94,460 with an average of 29,456 (see Figure 2). Over this period, peak count has had a significant decreasing trend (Kendall’s tau = -0.47, p < 0.05). The early period of record from 1986 to 2002 was highest, averaging 43,099 including the maximum peak count observation in 1989. Over these initial years of monitoring (1986-2002), the peak count was less than 20,000 for only one of the 17 years (6% of the years). From 2003-2024, the peak counts for 13 of the 21 years (59% of the years) were below 20,000. From 2003 to 2011, the observed peak count was notably lower and had less variability than previous years, with an average of 15,268 (range 12,375 to 24,000). This low population period followed unregulated horseshoe crab bait harvests in the mid-to-late-1990s. From 2012 to 2024, the stopover population increased to an average peak count of 21,439 red knots (range 6,880 to 32,930), which is an improvement over the previous nine years but is still 77 percent below the historic maximum count of 94,460 individuals in 1989. In 2021, the peak number of red knots (6,880) and ruddy turnstones (10,785) were the lowest recorded in 36 years of survey. Other shorebird species also occurred in lower numbers. All shorebirds were nearly absent from high-use beaches on the Cape May bayshore (north Reeds Beach to northern Norbury’s Landing, NJ) and low numbers were also observed on northern bay beaches (Thompson’s Beach, Moore’s Beach) despite widely available horseshoe crab eggs. A statistical population estimate, derived from resighting marked red knots, has fluctuated around 45,000 individuals since the method was implemented in 2011.8,9
Observations of peregrine falcon flyovers of shorebird flocks have increased in Delaware Bay over the last decade as the eastern U.S. peregrine population has recovered. Peregrine falcons hunt small birds and while their behavior of flushing shorebirds while hunting is natural, it poses a disturbance that can disrupt shorebird use of otherwise suitable habitats near peregrine nesting territories.10,11 In Delaware Bay, peregrine falcon nest structures have been removed to reduce this threat, however, non-nesting, sub-adult peregrines, not attached to any nest site, are naturally drawn to shorebird concentrations wherever they occur. To learn more about peregrine falcons, see our Environmental Trend Report about them.
Since 1997, data collection has included determining a proportion of banded red knots reaching a departure weight of at least 180 grams by May 26-28. These data are variable and show no significant trend over time. However, there is a significant increasing trend between the proportion reaching 180 grams and peak count. This trend may indicate that red knots are more likely to be observed in years that sufficient egg resources produce individuals with higher weights.
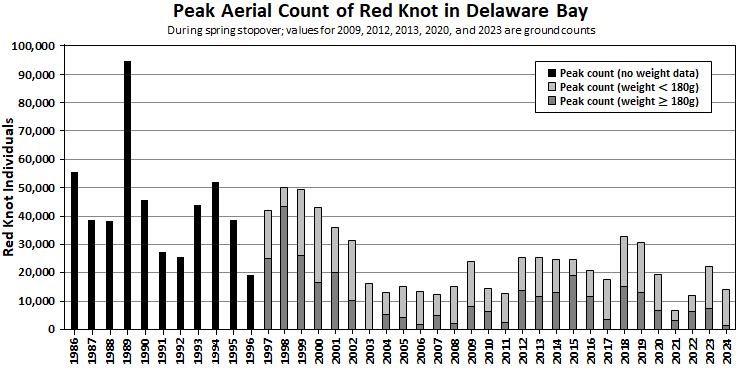
Figure 2. Peak Aerial Count of Red Knot in Delaware Bay
*Black bars show peak counts only, because from 1986-1996 data collection did not include bird banding efforts that include weight data. The bars for 1997-2024 are split into two categories: light gray shows the estimated proportion of red knots weighing less than 180 grams; dark gray shows the estimated proportion of red knot individuals weighing at least 180 grams.
The red knot abundance in the main South American wintering areas in Tierra del Fuego, Chile, appeared to decline from 15,512 in 2010 to 12,951 in 2024.12 Since 2010, this wintering population has fluctuated annually between ~10,000 and 15,000 individuals. As recently as 2000, however, this single wintering site held the majority of the Western Hemisphere’s red knots (>51,000).13 This wintering population, comprised of long-distance migrants, has declined most rapidly; 74% in 20 years. Overall, a hemispheric population estimated at 160,000 individuals in the mid-1980’s may number just about 46,000 as of September 2024.9,14,15
Horseshoe Crab Egg Densities
The weight of evidence suggests that the loss of horseshoe crab egg resources in Delaware Bay since the late 1990s has caused the observed decline in the red knot stopover population.6,16 Both horseshoe crabs and red knots have declined significantly since monitoring began.17,18 Five other shorebird species that rely on the Delaware Bay have also been declining during this time period.19
Scientists hypothesize that a minimum of 50,000 eggs/m2 is necessary to begin red knot recovery (see Figure 3).20 Since egg density data collection began in 1985, the average for New Jersey beaches has only surpassed this hypothetical minimum density one time, in 1991. The highest average egg density since then occurred in 2012 with over 25,000 eggs/m2, which resulted from particularly high densities at one site (Moore’s Beach). From 1985-2024, only 12% of monitoring years had an average egg density greater than 20,000 eggs/m2. The persistent low egg densities may explain why the egg density data does not have a significant relationship with peak counts or the proportion of red knots that reach at least 180 grams. Consistent horseshoe crab egg density data were not collected until 1996, during the period of unregulated horseshoe crab bait harvesting. If egg density were to reach a consistently higher density, the hypothesized recovery trend could potentially emerge.
Average egg densities located in the top 5 cm of sand remain low in recent years, with 2024 averaging 7,618 eggs/m2. One location in Mispillion Harbor, DE, is an exception to the overall low egg densities in Delaware Bay. This area has protection from all weather, and earlier warming of water which favors consistently high densities of spawning crabs and surface eggs– more than one to two orders of magnitude larger than mean egg densities on all other Delaware and New Jersey beaches. While Mispillion Harbor is very important, it can support only a portion of red knots and other shorebirds that come to the Bay.
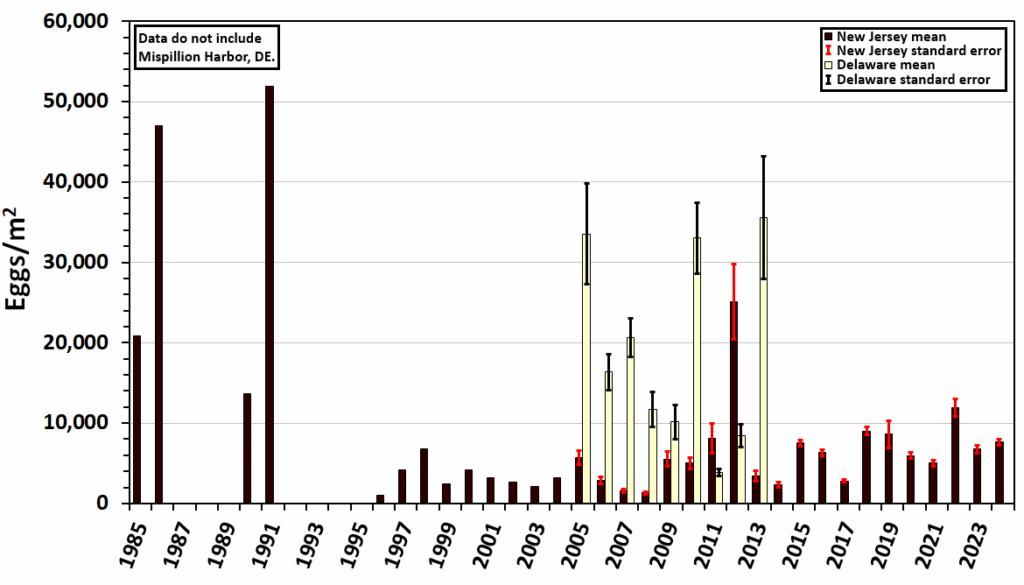
Figure 3. Mean Horseshoe Crab Egg Densities, Delaware and New Jersey
*While included in the figure, the sampling methodology from 1985-2004 differs from the later period, but may not be directly comparable. Data collected from 1985-1999 are from Botton and Loveland (unpublished data); data from 2005 to 2024 are from NJDEP Endangered and Nongame Species Program (unpublished data).
Outlook and Implications
Most biologists agree that the red knot population will take time to recover because of normally low reproductive rates associated with Arctic-breeding shorebirds. Successful reproduction requires several factors to be favorable in a given year, including food resource availability, Arctic conditions, and timing.21 Red knots are relatively long lived, and their populations can be sustained with intermittent years of less successful reproduction. But the fat stores from Delaware Bay are a key element to adult survival and reproductive success for red knots passing through New Jersey. Modeling efforts have indicated that there is potential for a rapid recovery of horseshoe crabs and their eggs with sufficient management22, which could allow the majority of red knots to maximize productivity, stabilize, and begin population recovery.
Projects to determine annual red knot population status are ongoing. These projects include collection of key data for long-term model development, horseshoe crab egg counts, shorebird weight surveillance, and individual marking of red knots for annual survival and population estimates.

Red Knot in flight (Getty Images)
Status of horseshoe crabs
Management efforts by the states and the Atlantic States Marine Fisheries Commission (ASMFC) have led to a decrease in the harvest of horseshoe crabs in Delaware Bay, from over 2 million per year in the late 1990s to approximately 550,000 per year from 2009-2023.23,24 The New Jersey Ocean Trawl Survey has also shown a changing trend in mean catch per unit effort, decreasing from 1988 to 2010 and then increasing from 2011 to 2023 (see our Horseshoe Crab Environmental Trend Report for more detail). This trawl survey was designed and sanctioned by ASMFC Technical Committees.
American horseshoe crabs have been classified as “Vulnerable” reflecting an elevated risk of extinction globally.25 However, Delaware Bay populations have been increasing since 2012, according to the latest stock assessment update, which is largely attributable to management efforts.26 On March 25, 2008, in an effort to increase horseshoe crab populations, the New Jersey legislature passed and then New Jersey Governor Corzine signed legislation (N.J.S.A. 23:2B-21) enacting a moratorium on the harvesting of horseshoe crabs that is to remain in place until the populations of both horseshoe crabs and red knots have returned to a level where they will be self-sustaining as determined by a peer-review panel of qualified shorebird and horseshoe crab ecologists and the Endangered and Nongame Species Advisory Committee. However, it is increasingly clear that without significant additional reduction of horseshoe crab mortality, particularly mature females, from various sources including bait harvest, lysate production, by-catch and illegal harvest, unregulated harvest in federal waters, horseshoe crabs and their eggs are not likely to increase in the short-term, and this continues to jeopardize red knot recovery. Of equal concern, the fisheries for which horseshoe crabs are used as bait, American eel and Whelk, are themselves overfished. American eel is classified by ASMFC as a “depleted stock”.27 While the Whelk fishery remains unregulated, the ASMFC raised concern over the dramatic increase in coastwide Whelk landings (all four species) since 2005.28 Like horseshoe crabs, whelks have slow growth and extended age to sexual maturity making them highly sensitive to overfishing.25
Management Programs
In an effort to offset the continued impact on the red knot, biologists have initiated several management programs to improve conditions for red knots and horseshoe crabs, primarily stewardship to minimize human disturbance to feeding shorebirds during the month of May. In December 2024, a proposal for a new shore protection rule was announced (N.J.A.C. 7:25-4A). This regulation would provide a process for the Department to establish when a restriction of public access to tidal waters and shorelines is necessary to protect threatened or endangered species, as well as provide for enforcement of such beach restrictions. If approved, the rule may go into effect during the summer of 2025.
Restoration of horseshoe crab spawning habitat has been ongoing since 2013, including removing rubble from beaches, adding large quantities of sand for beach replenishment, and revegetating marsh habitat. Continued restoration efforts are expected to reduce erosion, improve spawning habitat and increase egg resources for red knots.
Climate Change
With an annual interhemispheric journey from wintering to breeding ground, climate change is likely to impact red knots and there is evidence that climate change impacts on the species are already occurring (see U.S. Fish & Wildlife Service’s Rufa Red Knot Species Status Assessment Report).21 Along their journey, warmer coastal waters in the Delaware Bay could affect the timing of horseshoe crab breeding, and in turn cause red knots to miss the key opportunity to rapidly gain weight by feeding on crab eggs. As described above, if red knot weights are too low when departing Delaware Bay for the Arctic, it may lead to increased mortality and reduced reproduction rates. Additionally, their Arctic breeding habitat is subject to warming rates that are at least two times greater than the global surface temperature.29 Temperature changes in the Arctic may have several impacts, including reduced suitability of their nesting habitat, missed timing for peaks of key food resources, and increases in predation.21
More Information
Red Knots:
- Rufa Red Knot (Calidris canutus rufa) – S. Fish & Wildlife Service
- Recovery Plan for the Rufa Red Knot (Calidris canutus rufa) – U.S. Fish & Wildlife Service
- Red Knot Overview, All About Birds – Cornell Lab of Ornithology
Migratory Shorebirds:
https://njdepwptest.net/njfw/conservation/delaware-bay-shorebirds/
http://www.conservewildlifenj.org/protecting/projects/shorebird/
Beach Restoration for Shorebird and Horseshoe Crab Spawning:
https://www.littoralsociety.org/beach-restoration.html
https://www.nfwf.org/media-center/videos/ready-red-knots
More information on horseshoe crabs is available in “Wildlife Populations: Horseshoe Crab” in the NJDEP Environmental Trends series at https://njdepwptest.net/environmental-trends.
Inquiries regarding red knot survey data should be directed to:
Endangered & Nongame Species Program, Fish and Wildlife, via email at Nongame@dep.nj.gov.
Suggested Citation
NJDEP. “Wildlife Populations: Red Knot.” Environmental Trends Report, NJDEP, Division of Science and Research. Last modified June 2025. Accessed [month day, year]. https://njdepwptest.net/dsr/environmental-trends/red-knot/.
Download the Data
The data used in Figures 2 and 3 is available to download here.
References
1Endangered and Threatened Wildlife and Plants; Threatened Species Status for the Rufa Red Knot; Final Rule, 79 Fed. Reg. 73705 (December 11, 2014). http://www.fws.gov/policy/library/2014/2014-28338.html), Accessed April 2025.
2U.S. Fish and Wildlife Service. 2023. Recovery plan for the rufa red knot (Calidris canutus rufa). U.S. Fish and Wildlife Service, Northeast Region, Hadley, Massachusetts. 22 pages.
3Beans, B. E., and L. Niles, eds. 2003. Endangered and Threatened Wildlife of New Jersey. Rutgers University Press, New Brunswick, NJ.
4D’Amico, V.L., M.N. Bertellotti, A.J. Baker, W.R. Tellion, Jr., and P.M. Gonzalez, 2008. Migration Strategies of Wintering Populations of Red Knots Calidris canutus rufa in South America: The Role of Parasite Pressure. Ardeola 55(2): 193-202.
5Dey, A., M. Danihel, L. Niles, K. Kalasz, G. Morrison, B. Watts, H. Sitters. 2014. Update to the Status of Red Knot (Calidris canutus rufa) in the Western Hemisphere, August 2014. Report provided to the US Fish and Wildlife Service and the Atlantic States Marine Fisheries Commission.
6Baker, A. J., P. M. Gonzalez, T. Piersma, L. J. Niles, I. L. S. do Nascimento, P. W. Atkinson, N. A. Clark, C. D. T. Minton, M. K. Peck, G. Aarts. 2004. Rapid population decline in red knot: Fitness consequences of decreased refueling rates and late arrival in Delaware Bay. Proceedings of the Royal Society B 25:125-129.
7NJ Department of Environmental Protection, Division of Fish and Wildlife, unpublished peak aerial count data.
8Lyons, J. E., W. L. Kendall, J. A. Royle, S. J. Converse, B. A. Andres, and J. B. Buchanon. 2016. Population Size and Stopover Duration Estimation Using Mark-resight Data and Bayesian Analysis of a Superpopulation Model. Biometrics 72:262-271. https://doi.org/10.1111/biom.12393
9Lyons, J. E. Memorandum to the Delaware Bay Adaptive Resource Management (ARM) Working Group. Red knot Stopover Population Estimate for 2020. September 23, 2020. 12 pgs.
10Hope, D. D., D. B. Lank, P. A. Smith, J. Paquet and R. C. Ydenberg. 2020. Migrant Semipalmated Sandpipers (Calidris pusilla) have over four decades steadily shifted toward safter stopover locations. Front. Ecol. Evol. 8:3. doi: 10.3389/fevo.2020.00003
11Watts BD, B. R. Truitt. 2021. Influence of introduced peregrine falcons on the distribution of red knots within a spring staging site. PLoS ONE 16(1): e0244459. https://doi.org/10.1371/journal.pone.0244459
12Norambuena, H.V., R. Matus, E. Sandvig, A. Larrea, and C. Espoz. 2024. Aerial censuses of shorebirds in the Bahía Lomas Nature Sanctuary, January 2024. Technical report-Bahía Lomas Center, Santo Tomás University. 17 pp.
13Morrison, R.I. G. and R. K. Ross, 1989. Atlas of Nearctic shorebirds on the coast of South America. Special Publication, Canadian Wildlife Service, Ottawa, ON, Canada.
14Morrison, R.I.G., R.E. Gill, Jr., B.A. Harrington, S. Skagen, G.W. Page, C.L. Gratto-Trevor & S.M. Haig. 2001b. Estimates of shorebird populations in North America. Canadian Wildlife Service Occasional Paper no. 104. Canadian Wildlife Service, Ottawa.
15Atlantic States Marine Fisheries Commission. 2024. Delaware Bay Horseshoe Crab Harvest Recommendation for 2025. Memorandum to the Horseshoe Crab Management Board. 9pp. https://asmfc.org/wp-content/uploads/2025/02/DBETC_ARM_HSC2025HarvestRecommendation_Sept2024-1.pdf
16Duijns, S. et al. 2017. Body condition explains migratory performance of a long-distance migrant. Proc. R. Soc. B 284: 20171374. http://dx.doi.org/10.1098/rspb.2017.1374.
17NJ Department of Environmental Protection, Division of Fish and Wildlife, unpublished peak aerial count data.
18Zimmerman, J. Pers. Comm. 2019. Delaware Division of Fish and Wildlife, unpublished horseshoe crab trawl data; DE 30-foot trawl survey (Adult Index, April – July).
19Niles, L. J., J. Bart, H. P. Sitters, A. D. Dey, K. E. Clark, P. W. Atkinson, A. J. Baker, K. A. Bennett, S. Kalasz, N. A. Clark, J. Clark, S. Gillings, A. S. Gates, P. M. Gonzalez, D. E. Hernandez, C. D. T. Minton, R. I. G. Morrison, R. R. Porter, R. K. Ross, and C. R. Veitch. 2009. Effects of Horseshoe Crab Harvest in Delaware Bay on Red Knots: Are Harvest Restrictions Working? Bioscience 59:153-164. https://academic.oup.com/bioscience/article/59/2/153/228348
20NJ Department of Environmental Protection, Division of Fish and Wildlife, unpublished horseshoe crab egg density data.
21U.S. Fish and Wildlife Service. 2020. Species status assessment report for the rufa red knot (Calidris canutus rufa). Version 1.1. Ecological Services New Jersey Field Office, Galloway, New Jersey.
22Tan, Y., Jardine, S.L. 2019. Considering Economic Efficiency in Ecosystem-Based Management: The Case of Horseshoe Crabs in Delaware Bay. Environ Resource Econ 72, 511–538. https://doi.org/10.1007/s10640-017-0204-x
23Atlantic States Marine Fisheries Commission, Fishery Management Report No. 32, Interstate Fishery Management Plan for Horseshoe Crab, December 1998. 67 pp.
24Atlantic States Marine Fisheries Commission, 2024. Review of the Interstate Fishery Management Plan for Horseshoe Crab (Limulus polyphemus), 2023 Fishing Year. 33 pp.
25Smith, D.R., Beekey, M.A., Brockmann, H.J., King, T.L., Millard, M.J. & Zaldívar-Rae, J.A. 2016. Limulus polyphemus. The IUCN Red List of Threatened Species 2016: e.T11987A80159830. https://dx.doi.org/10.2305/IUCN.UK.2016-1.RLTS.T11987A80159830.en. Accessed April 2025.
26Atlantic States Marine Fisheries Commission (ASMFC). 2024. 2024 Horseshoe Crab Stock Assessment Update. Arlington, VA. 92pp. https://asmfc.org/resources/science/stock-assessment/horseshoe-crab-stock-assessment-update-2024/
27Anstead, K. August 2019 Presentation to the American Fisheries Society: “Assessing the Stock of American Eel: Past Results, Current Data Needs, and Future Goals”. http://www.asmfc.org/files/AmEel/2018AFS/Wednesday_309_1020_Kristen_Anstead.pdf
28Atlantic States Marine Fisheries Commission, 2013 Review of the Atlantic States Marine Fisheries Commission Fishery Management Plan for Horseshoe Crab (Limulus Polyphemus) 2012 Fishing Year. 28 pp. www.asmfc.org/uploads/file/hscFMPReview2013.pdf
29IPCC, 2021: Summary for Policymakers. In: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Masson-Delmotte, V., P. Zhai, A. Pirani, S.L. Connors, C. Péan, S. Berger, N. Caud, Y. Chen, L. Goldfarb, M.I. Gomis, M. Huang, K. Leitzell, E. Lonnoy, J.B.R. Matthews, T.K. Maycock, T. Waterfield, O. Yelekçi, R. Yu, and B. Zhou (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp. 3−32, doi:10.1017/9781009157896.001.