
Climate Science Basics
Climate change poses a real, immediate, and growing threat to New Jersey’s future.
The earth’s average temperature has risen by 1.5°F over the past century, and is projected to rise another 0.5 to 8.6°F over the next hundred years. Click below to learn the difference between weather and climate, why climate change is occurring and its impacts.
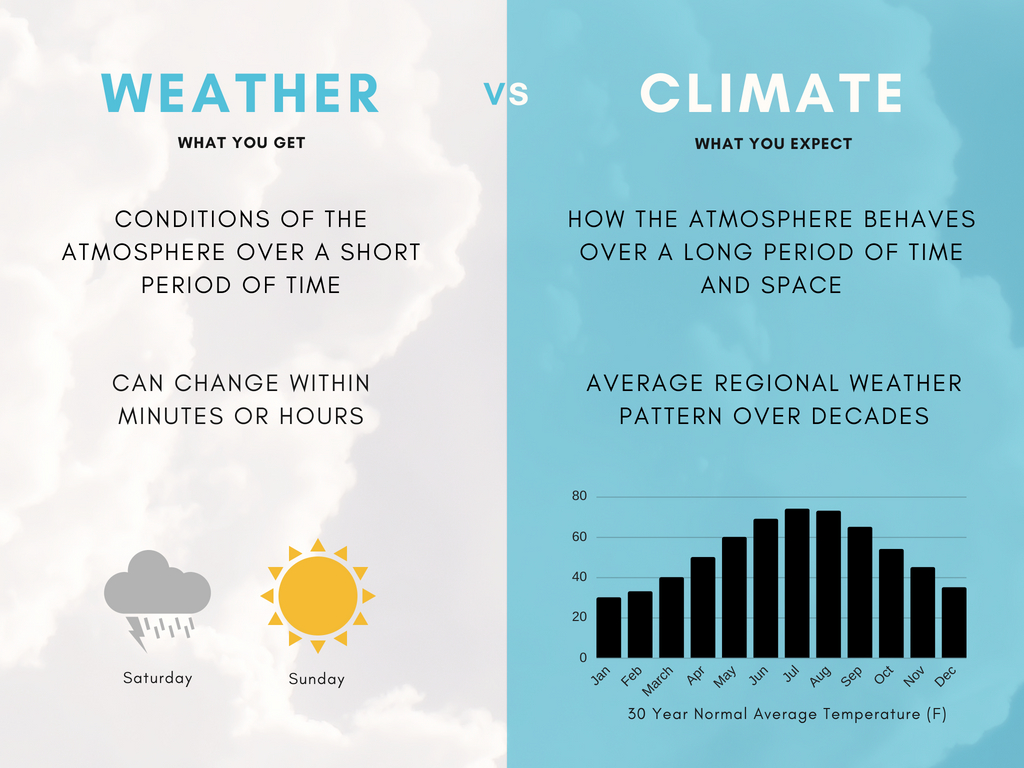
The difference between weather and climate is a measure of time. While weather is what conditions of the atmosphere are over a short period of time (from minutes to months), climate is how the atmosphere ”behaves“ over time and space. In short, climate is the description of long-term patterns of weather in a particular area. An easy way to remember the difference is that climate is what you expect, like a very hot summer, and weather is what you get, like a hot day with pop-up thunderstorms!
Described by familiar weather terminology such as temperature and precipitation, climate across the United States ranges from hot-humid to subarctic-arctic, with New Jersey ranging between cold and mixed-humid. New Jersey has five distinct climate regions, Northern, Central, Pine Barrens, Southwest, and Coastal. The geology, distance from the Atlantic Ocean, and prevailing atmospheric flow patterns produce the variations in daily weather between each region. To learn more about the climate of New Jersey visit the Office of the New Jersey State Climatologist.
Historical weather data, when aggregated with many indirect measures of climate such as ice cores, tree rings, glacier lengths, pollen remains, and ocean sediments, and by studying changes in the Earth‘s orbit around the sun, allows climate scientists to reconstruct past climates, and, until recently, established a basis for understanding future climate patterns.
Sources
What is Climate Change?
Climate Change refers to a significant change in the measures of climate (like temperature, precipitation, wind patterns, among other effects) lasting for an extended period of time.
Climate change is any significant change in the measures (otherwise known as indicators) of climate lasting for an extended period. Earth’s climate is naturally influenced by solar energy, and other earth systems, like the atmosphere, oceans and land cover, and natural changes in climate resulting from the interactions between the systems have happened over time. However, recent changes in the climate, over the last century, are due primarily to “man-made” influences, and it is this most recent “climate change” that is the focus of concerns for the future of the planet.
Key climate indicators include major changes in temperature, precipitation, extreme weather events and sea level rise, that occur over several decades or longer. Observations of these indicators across the United States and world provide multiple, independent lines of evidence of climate change caused by increasing concentrations of greenhouse gases (GHGs) in the atmosphere.
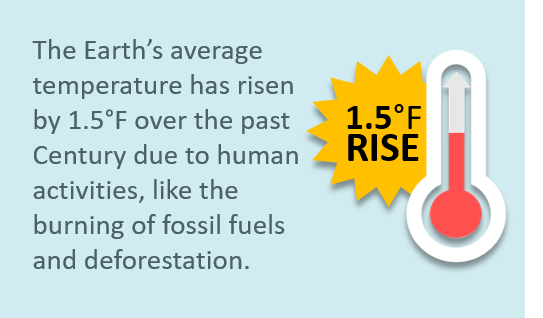
The earth’s average temperature has risen by 1.5°F over the past century, and is projected to rise another 0.5 to 8.6°F over the next hundred years. This phenomenon is colloquially referred to as global warming. Small changes in the average temperature of the planet can translate to large and potentially dangerous shifts in climate and weather, such as changes in rainfall, resulting in more floods, droughts, or intense storms, as well as more frequent and severe heat waves. The planet’s oceans and glaciers have also experienced big changes – oceans are warming and becoming more acidic, ice caps are melting, and sea levels are rising. As these and other changes become more pronounced in the coming decades, they will likely present challenges to our society and our environment. To learn more about what these indicators show for New Jersey, click on NJ Climate Indicators.
Climate change affects everyone. A warming climate brings changes that can affect our water supplies, agriculture, power and transportation systems, the natural environment, and even our own health and safety. Some of these changes are unavoidable, and although it’s difficult to predict the exact impacts of climate change, the climate we are accustomed to is no longer a reliable guide for what to expect in the future. However, scientists agree that there are ways to avoid the most dangerous climate impacts, and reduce the risks from a changing climate, that are both available and affordable. Our decisions today will shape the world our children and grandchildren will live in. Click on Take Action for more information on what you can do to address climate change in New Jersey.
What Is Causing Recent Climate Change?
Human activities are largely responsible for recent climate change. Over the past century, the burning of fossil fuels to produce energy, has released large amounts of carbon dioxide (CO2) into the atmosphere. Other human activities, such as deforestation, industrial processes, and some agricultural practices also emit greenhouse gases into the atmosphere. greenhouse gases are positive forcing because they absorb energy radiating from the Earth’s surface, rather than allowing it to be directly transmitted into space. This traps energy close to the surface of the Earth, acting like a blanket that warms the planet. This phenomenon, known as the greenhouse effect, is natural and necessary to support life on Earth. However, the ever-increasing amounts of greenhouse gases over the past century have increased this warming of the Earth’s climate, resulting in dangerous effects to human health and welfare, and to ecosystems. NOAA’s Annual GHGIndex, which tracks changes in radiative forcing from greenhouse gases over time, shows that such forcing from human-added greenhouse gases has increased 27.5 percent between 1990 and 2009. Increases in CO2 in the atmosphere are responsible for 80% of the increase. The contribution to radiative forcing by CH4 and CFCs has been nearly constant or declining, respectively, in recent years.
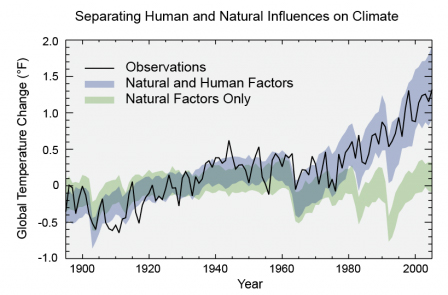
Source: U.S. National Climate Assessment (2014) adapted from Huber and Knutti
Scientists have pieced together a record of Earth’s climate by analyzing a number of indirect measures of climate such as ice cores, tree rings, glacier lengths, pollen remains, and ocean sediments, and by studying changes in the Earth’s orbit around the sun. This record shows that the climate system varies naturally over a wide range of time scales. In general, climate changes prior to the Industrial Revolution in the 1700s can be explained by natural causes, such as changes in solar energy, and volcanic eruptions, causing atmospheric CO2concentrations to vary within a range of about 180 to 300 parts per million by volume (ppmv). Warmer periods coincide with periods of relatively high CO2concentrations. Research indicates that these natural causes cannot explain the most observed warming since the mid-20th century. Rather, this warming is attributed to green house gas emissions from human activities
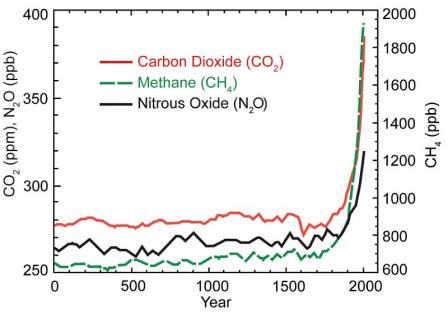
Source: U.S. National Climate Assessment (2014)
The graph above shows the increase in greenhouse gas concentrations in the atmosphere over the last 2,000 years. Increases in concentrations of these gases since 1750 are due to human activities in the industrial era. Concentration units are parts per million (ppm) or parts per billion (ppb), indicating the number of molecules of the greenhouse gas per million or billion molecules of air.
Source : USEPA https://archive.epa.gov/epa/climate-change-science/causes-climate-change.html#ref2
More Resources
Sources
- IPCC (2013). Climate Change 2013: The Physical Science Basis.Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change[Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- USGCRP (2014).Climate Change Impacts in the United States: The Third National Climate Assessment. [Melillo, Jerry M., Terese (T.C.) Richmond, and Gary W. Yohe, Eds.]U.S. Global Change Research Program.
- NRC (2010) Advancing the Science of Climate Changes. National Research Council. The National Academies Press, Washington, DC, USA.
- USEPA website at https://archive.epa.gov/epa/climate-change-science/causes-climate-change.html#ref2.
- Huber, M., and R. Knutti, 2012: Anthropogenic and natural warming inferred from changes in Earth’s energy balance. Nature Geoscience, 5, 31-36, doi:10.1038/ngeo1327
What are Greenhouse Gases?
Greenhouse gases (GHGs) are those gases that trap heat in the atmosphere, including carbon dioxide (CO2), methane (CH4), nitrous oxide ( N2O), and fluorinated gases (hydrofluorocarbons, perfluorocarbons, sulfur hexafluoride, and nitrogen trifluoride). Except for the fluorinated gases, these gases have emitted from both natural and human activities.
![]() Carbon dioxide
Carbon dioxide
Carbon dioxide (CO2) is the primary greenhouse gas contributing to recent climate change. CO2 is absorbed and emitted naturally as part of the carbon cycle, through plant and animal respiration, volcanic eruptions, and ocean-atmosphere exchange. Human activities, such as the burning of fossil fuels and changes in land use, release large amounts of CO2, causing concentrations in the atmosphere to rise. Atmospheric CO2 concentrations have increased by more than 40% since pre-industrial times, from approximately 280 parts per million by volume (ppmv) in the 18th century to over 400 ppmv in 2015. The U.S. Geological Survey (USGS) reports that human activities now emit more than 135 times as much CO2 as volcanoes each year. Human activities currently release over 30 billion tons of CO2 into the atmosphere every year.[2] The resultant build-up of CO2 in the atmosphere is like a tub filling with water, where more water flows from the faucet than the drain can take away.
![]() Methane
Methane
Methane (CH4) is produced through wetlands, agricultural activities, and fossil fuel extraction and transport. Methane is more abundant in Earth’s atmosphere now than at any time in at least the past 800,000 years.[2] Due to human activities, CH4 concentrations increased sharply during most of the 20th century, and are now more than two-and-a-half times pre-industrial levels. In recent decades, the rate of increase has slowed considerably.
![]() Nitrous oxide
Nitrous oxide
Nitrous oxide (N2O) is produced mainly through agricultural activities and natural biological processes. Fuel burning and some other processes also create N2O. Concentrations of N2O have risen approximately 20% since the start of the Industrial Revolution, with a relatively rapid increase toward the end of the 20th century. Overall, N2O concentrations have increased more rapidly during the past century than at any time in the past 22,000 years.
Other Greenhouse Gases
![]() Water Vapor
Water Vapor
Water vapor is the most abundant greenhouse gas, and also the most important in terms of its contribution to the natural greenhouse effect, despite having a short atmospheric lifetime. While some human activities can influence local water vapor levels, on a global scale, the concentration of water vapor is controlled by temperature, which influences overall rates of evaporation and precipitation. Therefore, the global concentration of water vapor is not substantially affected by direct human emissions.
![]() Tropospheric ozone (O3)
Tropospheric ozone (O3)
Tropospheric ozone (O3), which also has a short atmospheric lifetime, is a potent greenhouse gas. Chemical reactions create ozone from emissions of nitrogen oxides and volatile organic compounds from automobiles, power plants, and other industrial and commercial sources in the presence of sunlight. In addition to trapping heat, ground-level ozone is a pollutant that can cause respiratory health problems and damage crops and ecosystems. Ozone is the only National Ambient Air Quality Standard (NAAQS) that New Jersey has yet to attend. For more information on ozone as an air pollutant, visit http://www.nj.gov/dep/cleanairnj/
![]() Fluorinated gases
Fluorinated gases
Fluorinated gases such as chlorofluorocarbons (CFCs), hydrochlorofluorocarbons (HCFCs), hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6) are often used in coolants, foaming agents, fire extinguishers, solvents, pesticides, and aerosol propellants. Unlike water vapor and ozone, fluorinated gases have a long atmospheric lifetime, and some of these emissions will affect the climate for many decades or centuries.
Not All greenhouse gases Are Created Equal
While all greenhouse gases warm the Earth by absorbing energy and slowing the rate at which that energy escapes to space, different greenhouse gases have different effects on the Earth’s warming. Two key ways in which these gases differ from each other are their ability to absorb energy (their radiative efficiency), and how long they stay in the atmosphere (their lifetime). Global Warming Potential (GWP) allows direct comparisons of the global warming impacts of the different greenhouse gases. Specifically, it’s a measure of how much energy the emissions of 1 ton of a gas will absorb over a given time (usually 100 years), relative to the emissions of 1 ton of CO2. The larger the GWP, the more that a given greenhouse gas warms the Earth comparted to CO2. The table below list the different GWPs for the greenhouse gases.
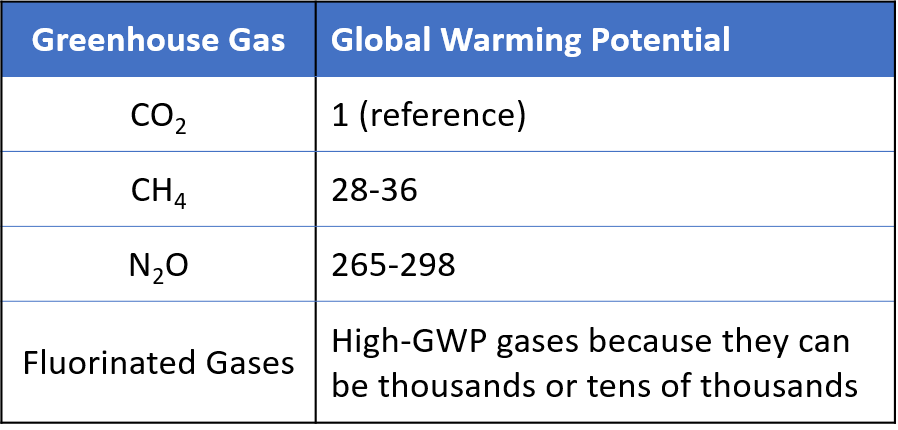
To learn more about GWP, visit https://www.epa.gov/ghgemissions/understanding-global-warming-potentials
So then what is carbon dioxide equivalent (CO2e)? This is a standard unit for measuring carbon footprint, by expressing the impact of various greenhouse gases on the climate. It describes, for a given mixture and amount of greenhouse gases, the amount of CO2 that would have the same global warming ability, when measured over a specified period (e.g., 100 years).
Sources
- IPCC (2013). Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Stocker, T.F., D. Qin, G.-K. Plattner, M. Tignor, S.K. Allen, J. Boschung, A. Nauels, Y. Xia, V. Bex and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
- USGCRP (2014). Climate Change Impacts in the United States: The Third National Climate Assessment. [Melillo, Jerry M., Terese (T.C.) Richmond, and Gary W. Yohe, Eds.] U.S. Global Change Research Program.
- NRC (2010). Advancing the Science of Climate Changes . National Research Council. The National Academies Press, Washington, DC, USA.
- USEPA website at https://archive.epa.gov/epa/climate-change-science/causes-climate-change.html#ref2.
Climate Change Glossary of Terms
A
Alternative Energy
Energy derived from nontraditional sources (e.g., compressed natural gas, solar, hydroelectric, wind).1
Atmosphere
The gaseous envelope surrounding the Earth. The dry atmosphere consists almost entirely of nitrogen (78.1% volume mixing ratio) and oxygen (20.9% volume mixing ratio), together with a number of trace gases, such as argon (0.93% volume mixing ratio), helium, radiatively active greenhouse gases such as carbon dioxide (0.035% volume mixing ratio), and ozone. In addition, the atmosphere contains water vapor, whose amount is highly variable but typically 1% volume mixing ratio. The atmosphere also contains clouds and aerosols.4
C
Carbon Dioxide (CO2)
A naturally occurring gas, and also a by-product of burning fossil fuels and biomass, as well as land-use changes and other industrial processes. It is the principal human caused greenhouse gas that affects the Earth’s radiative balance. It is the reference gas against which other greenhouse gases are measured and therefore has a Global Warming Potential of 1. 2
Carbon Dioxide Equivalent
A metric measure used to compare the emissions from various greenhouse gases based upon their global warming potential (GWP). Carbon dioxide (CO2) is the reference gas against which other greenhouse gases are measured and therefore has a Global Warming Potential (GWP) of 1. Carbon dioxide equivalents are commonly expressed as “million metric tons of carbon dioxide equivalents (MMTCO2Eq). The carbon dioxide equivalent for a gas is derived by multiplying the tons of the gas by the associated GWP.
MMTCO2Eq = (million metric tons of a gas) * (GWP of the gas)
Carbon Footprint
The total amount of greenhouse gases that are emitted into the atmosphere each year by a person, family, building, organization, or company. For example, a person’s carbon footprint includes all of the greenhouse gas emissions directly generated from that individual’s activities, such as by heating a home or riding in a car. It also includes greenhouse gases that come from producing the goods or services that the individual uses, including emissions from power plants that make electricity, factories that make products, and landfills where trash gets sent. Check out the EPA’s Carbon Footprint Calculator to estimate your carbon footprint.
Chlorofluorocarbons
Gases covered under the 1987 Montreal Protocol and used for refrigeration, air conditioning, packaging, insulation, solvents, or aerosol propellants. Since they are not destroyed in the lower atmosphere, CFCs drift into the upper atmosphere where, given suitable conditions, they break down ozone. These gases are being replaced by other compounds: hydrochlorofluorocarbons, an interim replacement for CFCs that are also covered under the Montreal Protocol, and hydrofluorocarbons, which are covered under the Kyoto Protocol. All these substances are also greenhouse gases.
Climate
Climate is the description of the long-term pattern of weather in a particular area. Climate is determined by looking at the averages of precipitation, temperature, humidity, sunshine, wind velocity, phenomena such as fog, frost, and hail storms, and other measures of the weather that occurs over a 30-year period in a particular place.
Climate Change
Any significant change in the measures of climate lasting for an extended period. In other words, climate change includes major changes in temperature, precipitation, or wind patterns, among other effects, that occur over several decades or longer. Current references to climate change are focused on those changes that are attributed to human activities, and might be mitigated through reduced production of CO2 emissions.
D
Deforestation
Those practices or processes that result in the conversion of forested lands for non-forest uses, such as agriculture or logging. Deforestation contributes to increasing carbon dioxide concentrations for two reasons: 1) the burning or decomposition of the wood releases carbon dioxide; and 2) trees that once removed carbon dioxide from the atmosphere in the process of photosynthesis are no longer present.1
E
Emissions
The release of a substance (usually a gas when referring to the subject of climate change) into the atmosphere.
Energy Efficiency
Programs or products aimed at using less energy to provide the same service.3
ENERGY STAR
A U.S. Environmental Protection Agency voluntary program that helps businesses and individuals save money and protect our climate through superior energy efficiency. Learn more about ENERGY STAR.
Energy Storage
The capture of energy produced at one time for use at a later time. Energy storage is particularly important for the long-term viability of certain renewable energies, such as wind and solar, which are intermittent.
F
Fossil Fuel
A general term for the organic materials (specifically, crude oil, coal, and natural gas) formed when decayed plants and animals are converted s by exposure to heat and pressure in the earth’s crust over hundreds of millions of years.1
Fluorinated Gases
Powerful synthetic greenhouse gases such as hydrofluorocarbons, perfluorocarbons, and sulfur hexafluoride that are emitted from a variety of industrial processes. Fluorinated gases are sometimes used as substitutes for stratospheric ozone-depleting substances (e.g., chlorofluorocarbons, hydrochlorofluorocarbons, and halons) and are often used in coolants, foaming agents, fire extinguishers, solvents, pesticides, and aerosol propellants. These gases are emitted in small quantities compared to carbon dioxide (CO2), methane (CH4), or nitrous oxide (N2O), but because they are potent greenhouse gases, they are sometimes referred to as High Global Warming Potential gases.
G
Global Warming
The recent and ongoing rise in global average temperature caused primarily by increasing concentrations of greenhouse gases in the atmosphere. Global warming is causing climate patterns to change. However, global warming itself represents only one aspect of climate change.
Global Warming Potential (GWP)
A measure of the total energy that a gas absorbs over a particular period of time (usually 100 years), compared to carbon dioxide.
Greenhouse Effect
Trapping and build-up of heat in the Earth’s atmosphere. Some of the heat flowing back toward space from the Earth’s surface is absorbed by water vapor, carbon dioxide, ozone, and several other gases (known collectively as Greenhouse Gases), and reradiated back toward the Earth’s surface. When the atmospheric concentrations of these greenhouse gases rise, the average temperature of the Earth’s lower atmosphere gradually increases. 1
Greenhouse Gas (GHG)
Any gas that absorbs infrared radiation in the atmosphere which includes carbon dioxide, methane, nitrous oxide, hydrochlorofluorocarbons, perfluorocarbons, sulfur hexafluoride, and other halogenated species that are listed in the IPCC. 1
H
Hydrochlorofluorocarbons (HCFCs)
Compounds containing hydrogen, fluorine, chlorine, and carbon atoms. Although ozone depleting substances, they are less potent at destroying stratospheric ozone than chlorofluorocarbons (CFCs). They have been introduced as temporary replacements for CFCs and are also greenhouse gases.
Hydrofluorocarbons (HFCs)
Compounds containing only hydrogen, fluorine, and carbon atoms. They were introduced as alternatives to ozone depleting substances in serving many industrial, commercial, and personal needs. HFCs are emitted as by-products of industrial processes and are also used in manufacturing. They do not significantly deplete the stratospheric ozone layer, but they are powerful greenhouse gases with global warming potentials ranging from 140 (HFC-152a) to 11,700 (HFC-23).
I
Indirect Emissions
Any greenhouse gas emissions from a building or electric vehicle that result from the electricity generation used. These emissions are called “indirect” because the actual emissions occur at the generation site, not at the location of the building or from the tailpipe of the electric vehicle using the electricity.
M
Methane (CH4)
A hydrocarbon that is a greenhouse gas with a global warming potential most recently estimated at 25 times that of carbon dioxide (CO2). Methane is produced through anaerobic (without oxygen) decomposition of waste in landfills, animal digestion, decomposition of animal wastes, production and distribution of natural gas and petroleum, coal production, and incomplete fossil fuel combustion.
Metric Ton
Common international measurement for the quantity of greenhouse gas emissions. A metric ton is equal to 2205 lbs or 1.1 short tons. 1
N
Nitrous Oxide (N2O)
A powerful greenhouse gas with a global warming potential of 298 times that of carbon dioxide (CO2). Major sources of nitrous oxide include soil cultivation practices, especially the use of commercial and organic fertilizers, fossil fuel combustion, nitric acid production, and biomass burning. The GWP is from the IPCC’s Fourth Assessment Report.
O
Ozone (O3)
Ozone, the triatomic form of oxygen (O3), is a gaseous atmospheric constituent. In the troposphere, it is created by photochemical reactions involving gases resulting both from natural sources and from human activities (photochemical smog). In high concentrations, tropospheric ozone can be harmful to a wide range of living organisms. Tropospheric ozone acts as a greenhouse gas. In the stratosphere, ozone is created by the interaction between solar ultraviolet radiation and molecular oxygen (O2). Stratospheric ozone plays a decisive role in the stratospheric radiative balance. Depletion of stratospheric ozone, due to chemical reactions that may be enhanced by climate change, results in an increased ground-level flux of ultraviolet (UV-) B radiation.
P
Perfluorocarbons (PFCs)
A group of chemicals composed of carbon and fluorine only. These chemicals (predominantly CF4 and C2F6) were introduced as alternatives, along with hydrofluorocarbons, to the ozone depleting substances. In addition, PFCs are emitted as by-products of industrial processes and are also used in manufacturing. PFCs do not harm the stratospheric ozone layer, but they are powerful greenhouse gases: CF4 has a global warming potential (GWP) of 7,390 and C2F6 has a GWP of 12,200. The GWP is from the IPCC’s Fourth Assessment Report. These chemicals are predominantly human-made, though there is a small natural source of CF4.
R
Recycling
Collecting and reprocessing a resource so it can be used again. An example is collecting aluminum cans, which are then melted down so the aluminum can be used in new aluminum products. 1
Renewable Energy
Energy from a source that is not depleted when used, such as wind or solar power.
Resilience
The ability to prepare for and adapt to changing conditions, and withstand and recover timely from disruptions.
S
Sequestration
The measurable transfer of carbon containing substances from the atmosphere or from a flux entering the atmosphere (such as power plant exhaust) by a physical, chemical, or biological process to a repository that is expected to contain and thus prevent the subsequent release to the atmosphere of 99 percent or more of that carbon for a period not less than 20 years.
Sink
Any process, activity or mechanism which removes a greenhouse gas, an aerosol or a precursor of a greenhouse gas or aerosol from the atmosphere. 4
Short Ton
Common measurement for a ton in the United States. A short ton is equal to 2,000 lbs or 0.907 metric tons.
Storm Surge
An abnormal rise in sea level accompanying a hurricane or other intense storm, whose height is the difference between the observed level of the sea surface and the level that would have occurred in the absence of the storm. 5
Sulfur Hexafluoride (SF6)
A colorless gas soluble in alcohol and ether, slightly soluble in water. A very powerful greenhouse gas used primarily in electrical transmission and distribution systems and as a dielectric in electronics. The global warming potential of SF6 is 22,800. This GWP is from IPCC’s Fourth Assessment Report.
W
Water Vapor
The most abundant greenhouse gas, it is the water present in the atmosphere in gaseous form. Water vapor is an important part of the natural greenhouse effect. While humans are not significantly increasing its concentration through direct emissions, it contributes to the enhanced greenhouse effect because the warming influence of greenhouse gases leads to a positive water vapor feedback. In addition to its role as a natural greenhouse gas, water vapor also affects the temperature of the planet because clouds form when excess water vapor in the atmosphere condenses to form ice and water droplets and precipitation.
Weather
Atmospheric condition at any given time or place. It is measured in terms of temperature, humidity, precipitation, cloudiness, brightness, visibility, wind, atmospheric pressure and other phenomenon. In most places, weather can change from hour-to-hour, day-to-day, and season-to-season.
Other Glossaries
US EPA Glossary of Climate Change Terms
United Nations Framework Convention on Climate Change Glossary
US Energy Information Administration Glossary
NASA’s Earth Observatory Library
National Weather Service Glossary
NJBPU Power Switch Glossary
Sources
- 1UNFCCC Glossary
- 2IPCC Glossary: Special Report on Renewable Energy Sources and Climate Change Mitigation (pdf)
- 3US Energy Information Administration Glossary
- 4IPCC Glossary: Third Assessment Report (pdf)
- 5US Climate Change Science Program. Coastal Sensitivity to Sea Level Rise: A Focus on the Mid-Atlantic Region (pdf)